All about heart pump Hawaii and the best interventional cardiologist in Hawaii
Best Interventional Cardiologist in Hawaii
Dr Ramy Badawi is one of the best interventional cardiologist. He is one of the leading mechanical circulatory support and Impella specialists in Hawaii. In 2011 he placed the first Impella 2.5 in Hawaii for cardiac arrest and participated in the implantation of the first Impella 5.0 in Hawaii for cardiogenic shock in 2012. Dr Ramy Badawi was involved in the first civilian transfer of a cardiogenic shock patient on mechanical circulatory support by air ambulance to the US mainland center for heart transplantation. He was instrumental in the introduction of the Impella RP device in Hawai’i in 2017 when it was first approved as a HUD by the FDA in the USA.
Up until today Dr Ramy Badawi is the only physician who maintains implanting privileges for mechanical circulatory support, specifically Impella, at several hospitals on O’ahu.
Heart pump Hawaii
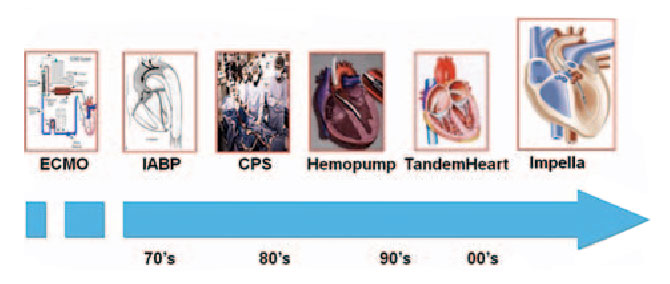
MCS
MECHANICAL CIRCULATORY SUPPORT (MCS)
This involves the use of a temporary heart pump to do the work of the heart to permit CHIP/CTO PCI and/or provide the body with a bridge to recovery.
Heart pump Hawaii
IABP
EMCO
Tandem Heart

Impella
Published on behalf of Eurpean Society of Cardiology
Heart pump Hawaii
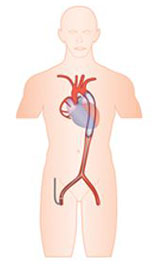
Intra-aortic balloon pump (IABP)
The IABP, first conceptualized by Harken in 1958 and then trialed by Moulopoulos at the Cleveland Clinic in the 1960’s, was first implanted by Adrian Krantowitz in 1968 and has existed in its current percutaneous form since the 1980’s. As such it is the oldest of the MCS systems and also one of the smallest in profile. It provides approximately 0.5 l/min of cardiac output.
Heart pump Hawaii
Heart pump Hawaii
Impella
Following on the Hemopump designed by Richard Wampler and using as its basis the Archimedes screw, the Impella heart pump devices were first FDA approved in the US in 2008 and are now the most commonly used percutaneous MCS after the IABP.
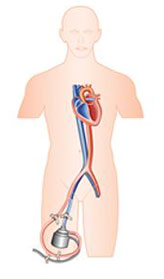
Tandem Heart
First conceptualized in 1991 this first in kind extracorporeal system was brought to market by Cardiac Assist Inc established in 1998 and began to see usage in the 2000’s. The Tandem Heart support system involved large bore cannulae which can be configured to support either the right of left heart depending on the placement of the inflow and outflow cannulae. It was first FDA approved as a portable device in 2006 and is now a product of Liva Nova which develops and sells a range of advanced circulatory support systems.
Heart pump Hawaii
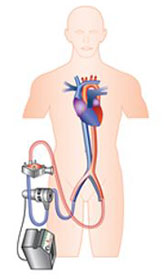
ECMO
Following on the Hemopump designed by Richard Wampler and using as its basis the Archimedes screw, the Impella heart pump devices were first FDA approved in the US in 2008 and are now the most commonly used percutaneous MCS after the IABP.
Reference
Abiomed
Liva Nova
Testimonials
Office Email
smasc1011@aol.com
Direct Email
cardiovascular.interventions.hi@gmail.com
Office Telephone
808 376 5340
Office Fax
808 625 4808
Heart pump Hawaii, Heart shock Hawaii, Impella Hawaii, High risk heart stent Hawaii, Protected PCI Hawaii, Cardiogenic shock Hawaii, Tandem Heart Hawaii, ECMO Hawaii, IABP Hawaii, Intra-aortic balloon pump Hawaii